From Discussions VOL. 12 NO. 1E-Fluid Induces Inflammation Responses in Lung Epithelial Cells
IN THIS ARTICLE
KEYWORDS
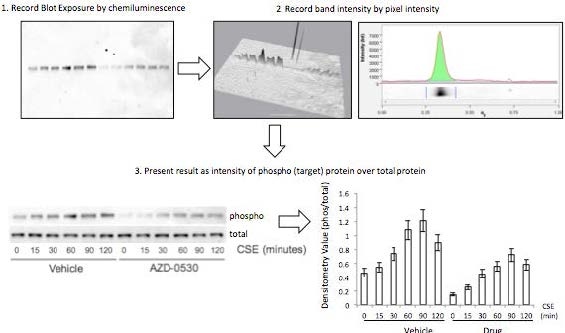
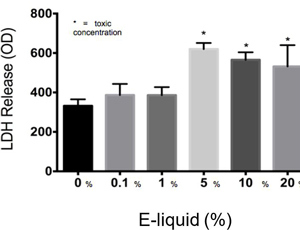
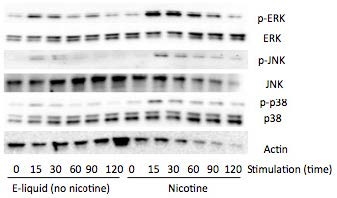
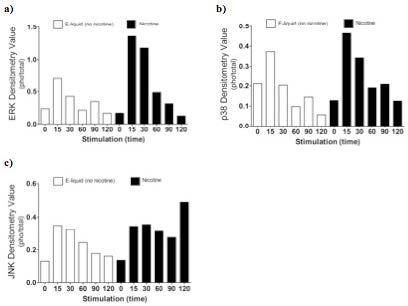
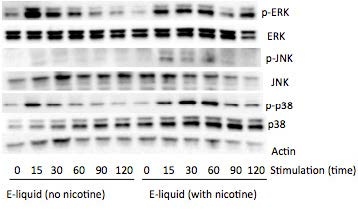
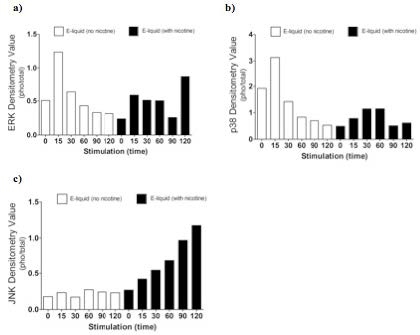
AbstractElectronic cigarettes (E-cigarettes) are devices that effectively deliver vaporized liquid nicotine to the lungs and are commercially available as a nicotine replacement therapy that is safer than conventional tobacco smoking. However, there is limited data available on the safety of E-cigarettes, and E-cigarettes currently do not fall under the regulation of the Food and Drug Administration (FDA). This study investigates the potential inflammation effects of E-cigarettes by utilizing lung cell culture techniques. Lung cells (A549 cell line) were exposed to the liquid that is vaporized by E-cigarettes (E-liquid), and toxicity and inflammatory markers were investigated. The E-liquid was found to be toxic to cells at concentrations greater than 2% E-liquid 0.72 mg/mL nicotine, 1% propylene glycol (PG), and 1% vegetable glycerin (VG) to media. Cells were also exposed to a nonlethal concentration of E-liquid, and the effects on kinase activation/induction {ERK, p38, and JNK) were examined. Conventional cigarette smoke activates these kinases (Mercer & D'Armiento, 2006, p. 137-50), which has been linked to the pathology of several lung diseases including chronic obstructive pulmonary disease (COPD). This study shows that when stimulated by E-liquid, these kinases undergo activation. Exposure to E-liquid also increases the activation levels of a specific target of kinases, NF-κB. Activation of NF-κB suggests that exposure to the contents of E-cigarettes could induce an inflammatory response in the lungs. Together, these findings highlight the potential harmful effects of E-cigarette exposure on the cells within the lung. IntroductionThere are projected to be more than a billion tobacco users on a global scale (Jha, 2006). Cigarette smoke exposure is the primary factor associated with disease initiation and progression for numerous diseases. Chronic obstructive pulmonary disease (COPD) is a progressive disease that induces airflow obstruction and is currently the third leading cause of death in the United States ("What is COPD", 2012). In the US, smoking prevalence has decreased over the past fifty years with approximately 19% of the population continuing to smoke. Therefore, several nicotine replacement therapy strategies have been implemented to aid smokers in quitting the habit. However, whether these strategies are a safer alternative for those individuals who cannot quit has yet to be determined. In an effort to find a safer replacement for smoking, many people have turned to electronic cigarettes (E-cigarettes). E-cigarettes are comprised of a battery and a replaceable cartridge with a propylene glycol or glycerin liquid solution containing differing quantities of nicotine. Upon inhaling, the subject breathes the vaporized liquid into the lungs. In contrast to traditional cigarettes, this apparatus releases far fewer toxicants (Pellegrino et al., 2012, p. 279-288) (Goniewicz, Knysak, & Gawron, 2013). However, recent studies performed in mice indicate that E-cigarette exposure could pose several future health problems. Exposures to E-cigarettes enhanced inflammation in mice (Lerner et al., 2015) and altered immune responses to microbial infection (Sussan et al., 2015). This study will test the inflammatory responses of epithelial cells to E-cigarettes. MethodsCell CultureHuman A549 cells (ATCC® CCL-185TM, ATCC, Manassas, VA), which are adherent lung epithelial cells, were grown in Dulbecco's Modified Eagle Medium (gibco® DMEM(1X) + GlutaMAXTM-1) supplemented with fetal bovine serum, penicillin, and streptomycin. Media was drained, phosphate-buffered saline (PBS) was used to rinse the container, and trypsin was added to the cells to sever cell adhesion to the flask. The resulting solution of cells and trypsin was transferred to a test tube and centrifuged for 5 minutes at 1100 rpm. Cells were re-suspended into fresh media and 2 mL of the resulting solution was pipetted into each well of a 6-well plate. Cells were allowed to adhere to the flask overnight to 60-80% confluency. These would be treated with PBS and liquid that is vaporized by E-cigarettes (E-liquid) with or without nicotine (inactive ingredients: propylene glycol (PG) and vegetable glycerin 50/50 (VG)). Prior to treating the cells, E-Liquid was diluted in cell media to the concentration labeled in each figure. Toxicity AssayTo determine the concentrations at which the E-cigarettes become toxic to cells, A549 cells were treated with the following concentrations of E-liquid without nicotine: 0, 0.1, 1, 5, 10, and 20%. PBS was used as a negative control. Cell death was examined by lactate dehydrogenase (LOH) release. Media was collected from cells 24 hours after performing the exposures. An LOH assay was performed, and 50 μL of each media sample was distributed to a well. Each sample was examined in triplicate to correct for pipetting errors. A 5 mL mixture containing equal volumes of LOH assay substrate solution, LOH assay cofactor, and LOH assay dye solution (1666.7 μL of each) was prepared. 50 μL (standard kit volume) of the prepared LOH mixture was pipetted into each well containing media, and the 96-well plate was placed in the dark for 30 minutes. The plate was then placed in the spectrophotometer at 410 nm to measure absorbance of the substrate. The rate of substrate turnover is measured by change in color from clear to pink/red and is proportional to LOH, as LOH is a marker for cell death. Stimulating the Cells with E-liquid at Timed IntervalsA549 cells were treated with 1% sub-toxic concentration of E-liquid (0 or 0.36 mg/mL nicotine, 0.5% PG, and 0.5% VG) at the following time points: 0, 15, 30, 60, 90, and 120 minutes (E-cigarette liquid is toxic to cells at a concentration of 5% or higher as shown by the results of the toxicity assay). In a sterile environment Qevel 2 bio-safety cabinet): nicotine and nicotine-free E-liquid (50%PG/50%VG) solutions were diluted to a final concentration of 1%, and nicotine was diluted to a final concentration of 0.01 mM. After 120 minutes, the media was drained from every well, PBS was used to rinse the wells, and 150 μL containing 27 μL of protease inhibitors, 27 μL of ethylenediaminetetraacetic acid, and 2700 μL of radioimmunoprecipitation assay (RIPA) lysis buffer were pipetted into each well. The prepared RIPA buffer solution caused the cells to burst so that the protein could be readily extracted. These protein samples were stored on ice. Western BlotsWestern blots were performed for the following targets: p-Erk, p p-38, p-JNK, p38, JNK, ERK, and actin. 10 μL of protein extract was mixed with 3 μL of 5X sample buffer in micro-centrifugation tubes and electrophoresed at 100 V for one hour. The extracts were transferred to a nitrocellulose membrane, which was rinsed with distilled water, Sigma® Ponceau S, and distilled water again. The nitrocellulose membrane was then placed in a 5% milk solution of 2.5 μL of lactose powder and 47.5 μL of PBS solution for one hour. The primary antibody was prepared using 4 μL of antibody solution and 4 mL of 2% bovine serum albumin (BSA). The nitrocellulose was placed in the antibody solution and incubated overnight with mild agitation. Following incubation, the nitrocellulose was rinsed thrice for 10 minutes each. It was then placed in a second antibody solution containing 4 μL of anti-rabbit horseradish peroxide (HRP) solution and 4 mL of 5% milk solution for two hours. For phosphorylated proteins, SuperSignal® West Femto Luminolj Enhancer Solution and SuperSignal® West Fem to Stable Peroxide Buffer (1,000 μL each) were mixed and pipetted onto the nitrocellulose membrane. For the total proteins, SuperSignal® West Pico Luminol Enhancer Solution, SuperSignal® West Pico Stable Peroxide Solution, SuperSignal® West Femto LuminoljEnhancer Solution, and SuperSignal® West Femto Stable Peroxide Buffer (500 μL each) were mixed and pipetted onto the nitrocellulose membrane. After five minutes, the nitrocellulose was placed in the Bio Rad Gel Dock Station. A chemi-luminescence camera was used to detect the HRP (secondary antibody). The amount of anti-rabbit HRP detected was proportional to the amount of protein present. Western blots were performed using antibodies that recognized the targets MMP-9, EGR1, TLR3, and p-Src 416. Densitometry AnalysisThe proteins on the nitrocellulose membrane were analyzed through densitometry analysis. A schematic representation of densitometry analysis is shown in Figure 1. Nuclear and cytoplasmic protein fractionationCells were processed with the compartment protein extraction kit (EMD Millipore) to isolate cytoplasmic and nuclear protein. Briefly, cells were mixed with 150 μL of cytoplasmic fraction buffer. The fraction was then incubated at 4 °C for 30 minutes (rotating). It was centrifuged at 10,000 rpm for 10 minutes at 4 °C. The pellet was isolated and washed with 1 mL PBS to remove any remaining cytoplasmic contents. The fraction was centrifuged again at 10,000 rpm for 10 minutes at 4 °C, and PBS was removed. The remaining nuclear pellet was lysed with 70 μL of nuclear buffer and incubated at 4 °C for 30 minutes (rotating). The nuclear solution was centrifuged at 14,000 rpm for 10 minutes at 4 °C. The supernatant was stored at -80 °C for later use and the nuclear pellet was discarded. Figure 1. Densitometry analysis was used to semi-quantify the level of activation of proteins by calculating a ratio of phosphorylated protein to total protein. The ratio was proportional to the level of activation of the protein. Also, total proteins were represented as a ratio of β-Actin. Transcription Factor Assay to Detect Activation of NF-κB and AP-1TransAMTM NF-κB, p65, and AP-1 (active motif) assay kits were used to detect and quantify transcription factor (NF-κB and AP-1) activation in nuclear fractions of cells. Cell extract containing activated transcription factor was added to the oligonucleotide coated plate. The activated NFKB subunit bound to the oligonucleotide plate was detected by using antibodies that attach to NF-κB/AP-1, p65, or c-jun AP-1 subunits. The secondary antibody conjugated to HRP was added to provide a sensitive colorimetric readout that was measurable by spectrophotometry. The amount of DNA binding was proportional to NF-κB activity. Then, 30 μL complete binding buffer was added to each well. 20 μL of nuclear extract sample diluted in lysis buffer was added to each well. 1 μL of Jurkat nuclear extract in 19 μL of complete lysis buffer was added as the positive control. 15 μL of lysis buffer was added as the negative control. The plate was incubated for one hour at room temperature (RT) with mild agitation (100 rpm). Each well was washed three times with 200 μL 1X wash buffer. 100 μL of diluted NF-κB antibody (1:1,000 dilution in 1X antibody binding buffer) was added to each of the 32 wells. The plate was covered and incubated for one hour at RT without agitation. The wells were washed thrice with 200 μL 1X wash buffer. 100 μL of diluted HRP-conjugated antibody (1:1,000 dilution in 1X antibody binding buffer) was added to all 32 wells and incubated as before. All wells were then washed four times with 200 μL 1X wash buffer. 100 μL developing solution was added to all wells. The plate was incubated for five minutes at RT in darkness until the sample wells turned from medium to dark blue. 100 μL of stop solution was added. In the presence of the acid, the wells turned yellow. Absorbance was read on a spectrophotometer within five minutes at 450 nm. ResultsThe LDH toxicity was performed to examine whether E-liquid induced airway epithelial cell death. Increased levels of LDH were observed in the media from cells exposed to greater than 5% E-liquid. Figure 2. The results of the LDH Assay show that E-liquid without nicotine is toxic to cells at a concentration of 5% or higher. Figure 3. Nicotine activates MAP kinases. Western blots of MAP kinases. Accordingly, cells were treated with a subtoxic concentration of 1% E-liquid following the data in Figure 2. Cells treated with 1% E-liquid were examined for MAP kinase activation, as these kinases are typically observed to undergo activation in lung diseases such as COPD and cancer. Western blots were performed for the following targets: p-ERK, p p-38, p-JNK, p38, JNK, ERK, and actin. The effects of1% E-liquid on MAP kinase signaling were examined. As indicated by the dark bands in Figure 3, MAP kinases were activated by the E-liquid without nicotine and nicotine alone. To semi-quantify the level of activation of MAP kinases, a ratio of phosphorylated to total proteins was calculated through densitometry analysis (Figure 4). ERK, JNK, and p38 were activated by nicotine alone. When treated with nicotine alone, ERK's densitometry value peaked at the 15-minute mark (Figure 4a). This ERK activation was prolonged over the 15 to 30 minute interval and steadily declined thereafter. When treated with nicotine alone, p38 has a more prolonged densitometry value at the 15 and 30-minute marks and a slow decline thereafter (Figure 4b). The densitometry value for JNK remained relatively stable and prolonged at the 15 and 30 minute marks (Figure 4c). JNK's densitometry value peaked at the 120-minute mark. Western blots were also performed to qualitatively assess the level of activation of the kinases induced by E-liquid containing nicotine. Figure 4. Semi-quantitative analysis of Western blots. Time O is a negative control. E-Iiquid (no nicotine) is a control. Nicotine activates (a) ERK, (b) p38, and (c) JNK. Figure 5. E-liquid with nicotine activates MAP Kinases. Western blots of MAP kinases. The level of activation of proteins induced by E-liquid with nicotine was semi-quantified by calculating a ratio of phosphorylated protein to total protein. When treated with E-liquid without nicotine, ERK•s densitometry value peaked at 15 and 120 minutes (Figure 6a). When treated with E-liquid without nicotine, p38 steadily rose in densitometry value from O to 30 minutes (Figure 6b). The densitometry value remained constant from 30 to 60 minutes. When treated with E-liquid containing nicotine, JNK steadily increased in densitometry value (Figure 6c). Increases in MMP-9. EGRt. TLR3, and p-Src 416 are associated with cigarette smoke exposure in individuals with COPD (Foronjy et al., 2008, p. 1149-57) (Reynolds. Cosio, & Hoidal. 2006, p. 3149). Western blots were performed to qualitatively assess their level of activation when treated with E-liquid containing nicotine. Cells were examined that had normal and silenced levels of a phosphatase PP2A. PP2A can block smoke induced inflammation and loss of PP2A results in greater lung inflammation (Wallace et al., 2012, p. 589-99). The darker bands in Figure 7 indicate a higher protein turnover induced by the E-liquid with nicotine. The densitometry bar graphs below were used to semi-quantify the levels of protein synthesis. Figure 6. Semi-quantitative analysis of Western blots. Time O is a negative control. E-liquid (no nicotine) is a control. (a) E-liquid with nicotine activates ERK. (b) E-liquid with nicotine activates p38. (c) E-liquid with nicotine activates JNK. Semi-quantitative analysis of the Western blot showed that there was little change in MMP-9 protein levels (Figure 8). However, silencing PP2A produced less of the protein MMP-9, as shown in Figure 8, while protein levels did increase in TLR3 and p-Src 416. Cigarette smoke increases EGR1 levels (Mercer & D'Armiento, 2006, p. 13750) and the data from Figure 8 suggests that E-liquid also increases EGRt levels. The effects of E-liquid containing nicotine were tested on NF-κB and AP-1. The transcription factor assay was performed to detennine whether E-liquid activated these transcription factors. NF-κB can undergo activation by MAP kinases (Jiang et al., 2003) and Src (Lee et al., 2007, p. 7001-11). EGRt also modulates NF-κB activity (Parra. Ferreira, & Ortega. 2011, p. 345-52). NF-κB is also activated by cigarette smoke in mice (Wallace et al. 2012, p. 589-99), and E-liquid containing nicotine strongly activated NF-κB. However, not all transcription factors are activated by E-liquid containing nicotine. There is no statistically significant difference in AP-1 activation following stimulation by E-liquid containing nicotine (Figure 9b).Continued on Next Page » Suggested Reading from Inquiries Journal
Inquiries Journal provides undergraduate and graduate students around the world a platform for the wide dissemination of academic work over a range of core disciplines. Representing the work of students from hundreds of institutions around the globe, Inquiries Journal's large database of academic articles is completely free. Learn more | Blog | Submit Latest in Biology |