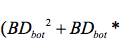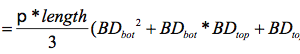From Center for Development and Strategy VOL. 2016 NO. 1Toxicological Effects of Nanomaterials on Aqueous and Terrestrial Ecosystems
IN THIS ARTICLE
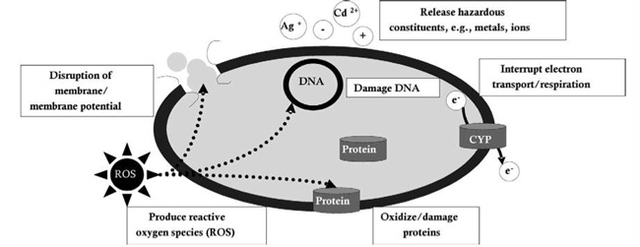
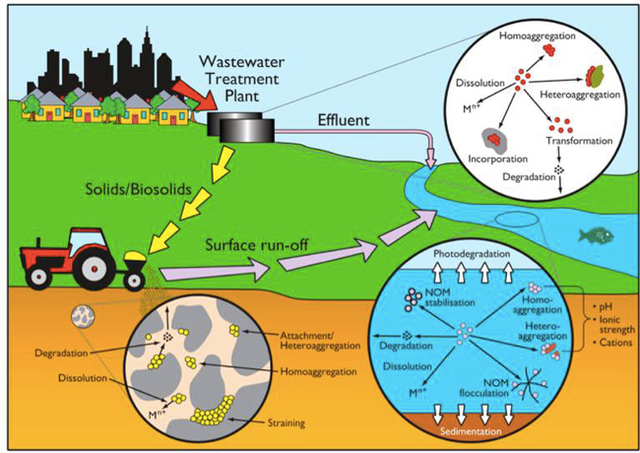
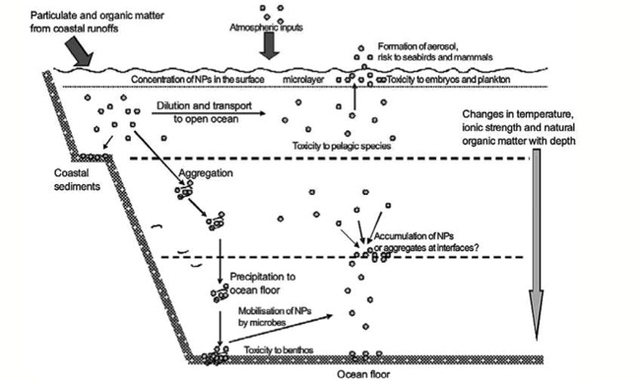
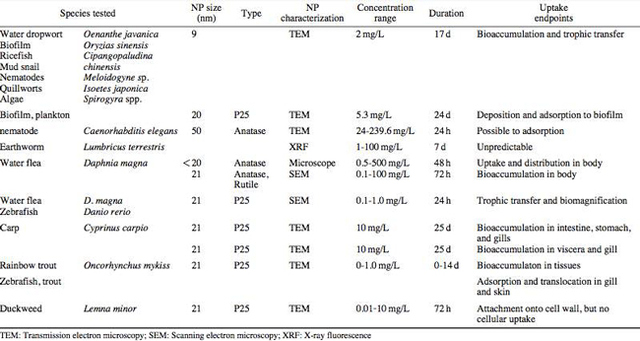
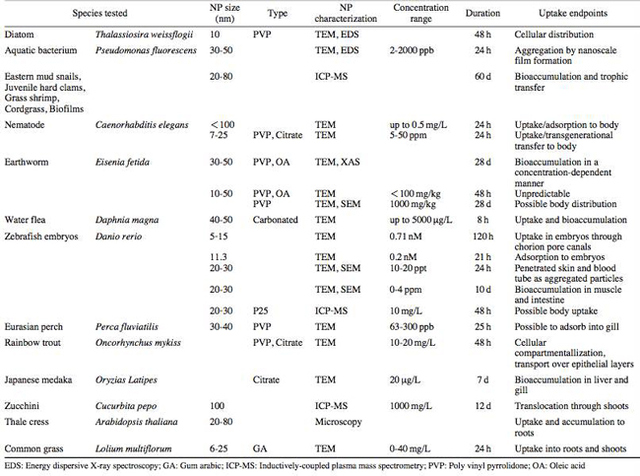
AbstractThis paper provides a holistic overview of the effects of engineered and natural nanomaterials (ENMs and NNMs) in the environment. Through increasing production and technology, NMs are contaminating the air, soil, and water with large toxicological implications. The proposed pathways of nanomaterials in the environment suggest that they accumulate in terrestrial and aquatic environment due to emissions, surface runoff and effluent from wastewater treatment facilities. Nanomaterial toxicological studies have also shown large bactericidal and mutagenic effects due to the metal ion properties, stabilizing agents and the size and shape of the NMs. (Suresh et al., 2013). These adverse effects that were seen on bacteria and microbes were also observed in plants and fish. Plants were seen to have inhibited growth, and even death when coming into contact with specific nanoparticles. (Burke et al., 2015) However, some conflicting data exist where plants have shown improvement in growth under specific exposure. (Lahiani et al., 2008) Fish and aquatic organisms were also studied in order to determine the health impacts on these species as well as the potential for biomagnification. Organ failure was observed in zebrafish and carp due to acute exposure to metal NPs (Griffit et al., 2011). Other harmful effects have also been observed in aquatic ecosystems, with many engineered nanoparticles settling in the sediment of freshwater and marine environments causing risk to benthic species (Handy et al., 2011). The impact on human health was also reviewed, revealing that the exposure to nanoparticles has lead to nausea, vomiting and even death. Human organs can also be severely affected by inhaling, ingesting or coming into contact with large amounts of nanomaterials. (Tang et al., 2015). IntroductionNanotechnology and nanomaterial (NM) containing products are part of a multi billion-dollar industry, with a wide variety of uses ranging from electronics to biomedical applications. Due to growing industry, it is inevitable that engineered and natural NMs will be released into the atmosphere, affecting both the water and soil. This could be from intentional or unintentional release, which means that it is vital to determine the potential impacts this growing industry can have on environmental, human, animal and plant health. Nanoparticles are naturally occurring in the environment, however through technological breakthroughs the abundance of new and engineered nanoparticles not found naturally are becoming prevalent in the atmosphere. Due to lack of knowledge there is no regulation on the emissions and many scientists are concerned that this could pose a threat to as a new class of environmental hazards. Nanomaterials are "structures with at least one dimension less than 100nm" which, due to their small size and large surface area NMs are widely used in various products, increasing the routes in which they can come in contact with organisms and plants. (Van Aken, 2015) (Hou. et al., 2013) Release of nanomaterials in the environment through emissions, industrial and commercial products can have a large effect causing them to end up in wastewater treatment plants and furthermore to surface water. (Hou. et al., 2013) The nanoparticles that are not filtered in wastewater treatment plants are most likely to "accumulate in benthic sediments" which can possibly cause problems for many species in lakes and rivers. (Batley. et al., 2011) Aquatic organisms are "particularly susceptible to pollutants due to their large, fragile, respiratory epithelium". (MacCormack. et al., 2008) Changes in pH, water temperature, oxygen levels can also increase the risks associated with nanomaterials in aqueous environments and must be considered when addressing risk. Plants are also susceptible to high exposure of nanomaterials through soil contamination or accidental release. Nanomaterials have been shown to enter living organisms and "exert toxic effects at the cellular level, including membrane disruption, protein inactivation, DNA damage, and disruption of energy transfer and release of toxic substances". (Van Aken, 2015) Due to the importance of plants and bacteria in the food chain it is crucial to understand the impact that this large industry could have in the future of environmental and human health. This paper will focus on the toxicological effects of waste engineered nanomaterials in terrestrial and aquatic environments and their implications on human health and environmental safety. BackgroundDefinition of NanomaterialsNanoparticles are naturally occurring in both aquatic and terrestrial environments in form of colloids, "mineral precipitates (Al, Fe, MgO, OH)" and dissolved organic matter". (Batley et al., 2011) The International Organization for Standardization (ISO) has classified three groups of nanomaterials. First, nanoparticles, which include all "three dimensions between 1 and 100 nm", are the most commonly referred nanomaterials. However, dimensionality plays a large role in determination of NM's showing that nanoplates (two dimensions between 1 and 100nm) and nanofibers (one dimension between 1 and 100nm) are also classified as NM's by ISO. (Batley et al., 2011) NNM's (natural nanomaterials) have always existed in the environment with little known toxicological effects. However, these definitions based only on size may not be sufficient in addressing toxicological risk, due to the fact that the nanomaterial version of a material exhibits different properties than it's nonnano counterpart. (Boverhof et al., 2015) With increasing nanotechnology and new products, engineered nanomaterials (ENM's) are now emitted into the air, water and soil. These ENM's can be categorized in "seven main classes: carbonaceous nanomaterials (carbon nanotubes (CNT's)); semiconductors (quantum dots); metal oxides (zinc oxide); nanopolymers (dendrimers); nanoclays; emulsions (acrylic latex); and metals (silver, gold)." (Buckley et al., 2011) Properties of Engineered Nanomaterials and Microbial ToxicitySuresh et al., (2013) have written a comprehensive review of the properties of nanomaterials and their relationship to microbial toxicity. Due to the fact that "microbial consortia underlie environmental processes" it is crucial to study the toxic effects of nanoparticles and the impacts this can have on "trophic balances". Nanomaterials are used in medicine because of their bactericidal and fungicidal properties, the most well known being Ag and CuO nanoparticles. (Suresh et al., 2013) However, when these engineered NM's are released into the environment such properties might have serious long-term impacts on both terrestrial and aquatic species. Some studies have tried to correlate the parent metal toxicity with that of its nano-form, however the size, shape, coating and the way the nanoparticle is synthesized determine that its toxicity is different than its parent metal. Furthermore, manufacturing processes add detergents, additives and other chemicals, which are not fully removed from the final product, increasing the toxicity. (Suresh et al., 2013) Metal nanoparticles are studied because they dissolve into ions in aquatic environments, which is "often a primary step and common cause for nanoparticle toxicity". (Suresh et al., 2013) The most widely studied and used ion for its microbial toxicity is silver, yet there are many studies showing that the "correlation between nanoparticle toxicity and that of its dissolved ion" is seen with other materials as well. (Suresh et al., 2013) Secondly, the coating of a nanomaterial determines the toxicity and effect it will have on bacteria. Usually, engineered nanoparticles are "surrounded by a shell or cap to act as a stabilizing, biocompatibility and or reacting agent". (Suresh et al., 2013) This shell can affect the charge of the nanoparticle and its interaction with the environment creating a completely different toxic effect than the nanoparticles parent material. Baumann et al., studied the acute effects on Daphnia Magna of four different types of coatings on iron oxide nanoparticles which stabilized the NPs yet had toxic effects on the organisms, including death. Lastly, the size and shape of a nanoparticle can have a large effect on bacteria "as the particle size decreases, the ratio of surface area to mass increases" resulting in changes to the "physical-chemical properties" of the nanoparticle. (Suresh et al., 2013) This creates novel applications due to "surface atom reactivity, electronic and optical properties" which can influence binding characteristics in bacteria and increased reactivity. (Suresh et al., 2013) A general trend of increased toxicity with a decrease in size has been observed due to increased reactivity of smaller particles. (Suresh et al., 2013) Below is a table highlighting the known microbial nanotoxicity studies. As previously mentioned; the coating, size, dosage and type of nanomaterial have different effects on different bacterial strains. Some surface coatings such as PEG and PVP are used as stabilizing agents for ZnO nanoparticles and display no known toxic effects on E. coli whereas an MES stabilized Ag+ ion displays inhibition in E. coli. PVP was also found to be the most promising stabilizer for medical applications due to its polymer coating, which reduces the release of iron ions through high colloidal stability. (Baumann et al., 2014) Most of the studied nanoparticles however, present possibilities of being bactericidal, mutagenic, cause membrane damage, act as inhibitors and destabilizers. Very few of the studied nanoparticles have no side effects in bacteria. Potential Mechanism of Biological Uptake and ToxicityKlein et al. have described some possible mechanisms of nanomaterial toxicity to bacteria, as shown below in Figure 1. Figure 1. A proposed schematic diagram by Klein et al., of the possible mechanisms of nanomaterial toxicity to bacteria. (Image retrieved from Klein et al., 2008) Nanoparticles are shown to enter the cell by diffusion through the membranes as well as through endocytosis and adhesion. (Klein et al., 2008) Quantum dots and carbon nanotubes (CNTs) are designed to interact with "proteins, nucleic acids or cell membranes for drug delivery purposes" which makes the unintentional interactions with the environment potentially hazardous. (Klein et al., 2008) As mentioned previously, CNTs are also some of the most mass produced nanoparticles for many applications, which increases the risk of environmental exposure through product use and industry leaks. The diagram depicts possible mechanisms, which include "disruption of membranes or membrane potential, oxidation of proteins, genotoxicity, interruption of energy transduction, formation of reactive oxygen species and release of toxic constituents." (Klein et al., 2008) These effects are all seen in the data provided in Table 1. of the known effects of nanomaterials on different bacterial strains. Discussion and AnalysisPathways of Nanomaterials in the EnvironmentNMs find their way into the environment intentionally and unintentionally, through consumer products such as medicinal devices, paints, electronics, cosmetics, car catalysts, plastics, ceramics and more. Other pathways could include industrial runoff, spills and waste. Most research is focused on ENMs that have a high potential for industrial spills or waste such as carbon nanotubes and metal oxides due to their mass production and possibly toxic effect on the environment. (Lowry et al., 2010) Some large risks associated with the manufacturing of these NMs are the unintentional leakage that can occur during the transportation of the NP's to secondary or tertiary sites. Kalavrouziotis et al., 2008) have studied the impact of the platinum group elements (Pt, Pd, Rh) emitted into the atmosphere through automobile catalyst converters. The study suggests that even though catalytic converters minimize the pollution emitted by the car exhaust fumes, the platinum group elements are being emitted in forms of particulate matter and accumulating in soil, plants and air. (Kalavrouziotis et al., 2009) Due to the nature of these emissions, the particulate matter is being transported over long distances and have "increased significantly during the last ten to fifteen years, especially along the road side of high ways". (Kalavrouziotis et al., 2009) Intentional releases of nanomaterials also occur when remediating contaminated soils, and the "use of iron NPs to remediate groundwater". (Klaine et al., 2008) Other pathways of intended release are through consumer goods, such as sunscreen, health care products, fabrics and paints, which enter the environment "proportional to their use". (Klaine et al., 2008) In order to effectively filter these NM's requires "a new class of nanostructured sorbents" which is not widely available due to unregulated emissions. Figure 2. Proposed schematic diagram of pathways of NPs into the environment. (Image retrieved from Batley et al., 2011) Despite knowledge of the pathways into the environment, trying to analyze the life cycle of the nanoparticles and the exact mechanisms of entry is quite challenging. Most studies have focused on in situ experiments, which can be vague and unlikely to show the correct processes. (Nam et al., 2014) However, "researchers have turned to microcosm and mesocosm systems as miniaturized ecosystems to simplify experimental conditions", which helps to understand "the uptake and bioaccumulation" of NPs. (Nam et al., 2014) Uptake of Nanoparticles in Aquatic EcosystemsSince nanoparticles are designed to "persist as particulate matter in aqueous media", they are able to pass through biological membranes due to their size. (Velzeboer et al., 2008) Nanoparticles in aqueous solutions form colloidal suspensions, which can potentially interact with aggregates, other colloidal suspensions and agglomerates. (Velzeboer et al., 2008) This occurs in marine ecosystems because they are generally more alkaline, have higher ionic strength and already have a "wide variety of colloids and natural organic matter". (Klaine et al., 2008) Coastal zones are very likely to have a high concentration of colloids, organic matter and nanoparticles, being closer to discharges or spills from plants or industries than deep ocean water. In freshwater, the nanoparticle aggregates have a high chance of sinking slowly to the bottom and accumulating in the sediment; this can have a negative effect on the benthic species. In marine ecosystems, it is possible that "nanoparticles will accumulate at the interface between cold and warm currents" which is not likely with freshwater. (Klaine et al., 2008) Another possibility for the mechanism of NPs in marine ecosystems could be a recycling through biota. This can increase the risk of the species, which feed within these cold and warm zones such as tuna. Lastly, Klaine et al, 2008 introduced another possibility, namely accumulation in the "surface microlayers of the oceans" where nanoparticles are trapped due to surface tension and viscous properties. This presents a risk to marine birds and mammals as well as the organisms living in the surface microlayer. (Klaine et al., 2008) However, there is no research that studies the different effects of accumulated nanoparticles in the surface microlayer of oceans. Figure 3. represents a schematic diagram of the proposed behavior of nanoparticles in marine ecosystems. It shows the nanoparticles and organic matter discharged from coastal runoff and suggests that the NPs are diluted and transported in the open ocean, and can also sink to the bottom and become aggregates. These aggregates are further transported by microbes or settle in sediment causing toxic effects to benthic species. Another mechanism shows that the free nanoparticles that are transported can have a toxic effect on pelagic species through direct contact, as well as become a risk to seabirds and mammals through aerosol formation. (Klaine et al., 2008). Figure 3. Schematic diagram showing the behavior and effects of NPs on marine environments as well as the organisms at risk of exposure. (Image retrieved from Klaine et al., 2008) Bioaccumulation of NMs in Aquatic EcosystemsNam et al., have written a comprehensive review, which focused on the bioaccumulation of NPs in aquatic environments. Due to the complexity of nanoparticles and the effects on the environment depending on the size, shape, coating and functionality, they decided to focus specifically on TiO2 NPs and Ag NPs. A simplified microcosm system was designed to "assess the bioaccumulation of TiO2 NPs in multiple model species". (Nam et al., 2014) They discovered that a high level of TiO2 NPs were present in the sediment layer due to settling of NPs as previously mentioned in the proposed schematic by Klaine et al. 2008. However, there was also experimental evidence, which showed a movement of the TiO2 from the sediment to "water dropwort roots and nematodes living on these plants". (Nam et al., 2014) Nanoparticles were also transferred through different trophic levels as shown by Nam et al., through transfer from "dropwort roots, to nematodes and snails feeding on these roots as well as from biofilmconsuming plankton to ricefish feeding on the plankton". (Nam et al., 2014) This shows that the engineered nanoparticles can travel through feeding patterns of aquatic organisms. There was also direct evidence of trophic transfer of TiO2 NPs showing NPs "transferred from water fleas to zebrafish, indicating potential biomagnification of TiO2 via food chain transfer". (Nam et al., 2014) Ag nanoparticles were also studied in order to measure the bioavailability of these nanoparticles to higher trophic organisms. (Nam et al., 2014) Nam et al., show that algal cells concentrate nanoparticles due to adhesion of NPs to the cell wall. They also provide a table summarizing the different uptake endpoints of the Ag NPs in different species. In the earthworm they found that the bioaccumulation depended on the concentration and could be possibly distributed throughout the body. In rainbow trout they propose that the possible adsorption occurs through the gill, which was similar to the Japanese medaka. (Nam et al., 2014) The two tables are presented below of both the bioaccumulation of TiO2 NPs as well as the Ag NPs. Table 2. Uptake and bioaccumulation of TiO2 nanoparticles in aquatic organisms (Table retrieved from Nam et al., 2014) Table 3. Uptake and bioaccumulation of Ag nanoparticles in aquatic organisms (Table retrieved from Nam et al., 2014) Impacts on Algae, Fish and Aquatic OrganismsThe toxic impact of nanomaterials on fish and aquatic organisms is important to study because most contaminants released in the environment are consumed by aquatic species. Griffitt et al., 2008 have conducted a study in order to assess the toxicity of metallic nanoparticles in aquatic organisms. They used zebrafish, daphnids and Pseudokirchneriella subcapitata as models of the varying trophic levels and different feeding strategies. These organisms were exposed to Cu NPs, Al NPs, Co NPs, Ag NPs, Ni NPs and TiO2 since they are the most commonly engineered nanoparticles with known parent metal toxicity. The results of the study showed that the nanometals were causing "acute toxicity in multiple aquatic species", and "filter-feeding invertebrates" having the highest susceptibility to metallic nanoparticle exposure. (Griffit et al., 2008) All the organisms tested were acutely susceptible to Ag NPs and Cu NPs, with the daphnids and algae being more affected by toxicity than zebrafish. (Griffit et al., 2008) Since daphnids are particulate filter feeders, they are more likely to be exposed to larger numbers of nanoparticles compared to larger organisms. (Griffit et al., 2008) These results also concluded that there was no apparent relation between size and surface area, and that the nanoparticles were capable of "causing acute toxicity in multiple aquatic species" regardless of their shape. (Griffit et al., 2008) Ag NPs and Cu NPs were the most toxic in all species yet both the size and shape of these nanoparticles varies greatly, and other nanomaterials with the same sizes have no effect on organisms. Furthermore, silver and copper are the most toxic when presented in a soluble form and the toxicity was confirmed to be partly because of dissolution of particles. (Griffit et al., 2008) These toxic effects however are not only limited to daphnids and MacCormack et al., suggest that the physical dimension of nanoparticles may allow them to "interact with cellular receptors or transport proteins" in aquatic animals. "The respiratory and ion transport surface area can be greater than 60% of the total surface area" of fish posing large health threats when interacting with nanoparticles. (MacCormack et al., 2008) It was found that the interaction of gill ion transport with metal nanoparticles resulted in ionoregulatory failure. (MacCormack et al., 2008) Furthermore, studies have mostly focused on the effects of NPs under "steady-state physiological conditions", which is not the case in aquatic environments. (MacCormack et al., 2008) Marine ecosystems have changes in pH, salinity, pressure and temperature, which is not taken into account when studying nanomaterial toxicity in animals living under these conditions. (MacCormack et al., 2008) Specifically, cell membrane structure is altered during temperature changes by the "variation of fatty acids and cholesterol" which can be affected by nanomaterials that are specifically engineered to insert "into biological membranes". (MacCormack et al., 2008) Salinity of marine ecosystems also has to be considered, as "fish adapt to changes in salinity" through changes in their gill membrane which also effects organisms "exposure to nanomaterials in the environment". (MacCormack et al., 2008) Lastly, due to limited oxygen exposure in water, "fish exhibit hypoxia" increasing their vulnerability of nanoparticle exposure and toxicity. Hypoxia is exhibited in forms of increased "ventilatory effort" as well as "cardiovascular changes necessary to exploit adjustments". (MacCormack et al., 2008) This increases the risk of toxicity to the respiratory system since many species double their skin capillary surface area in order to improve the efficiency of oxygen uptake, allowing for nanoparticles to enter the system and cause acute toxic effects in aquatic species. (MacCormack et al., 2008) Chronic exposure of nanomaterials to aquatic species is important to study in order to know long-term effects this will have on organisms. Zhu et al., have studied the chronic exposure to sublethal fullerenes aggregates on carp showing that the most susceptible organs were found to be the gills, the brain and the liver. They found that "oxidative stress due to long-term exposure could be the main mechanism for toxicity" for these fish under freshwater conditions. (Zhu et al., 2008) These findings are important in creating regulations that will penalize companies for spills or emissions of nanomaterials in rivers, lakes and oceans.Continued on Next Page » Suggested Reading from Inquiries Journal
Inquiries Journal provides undergraduate and graduate students around the world a platform for the wide dissemination of academic work over a range of core disciplines. Representing the work of students from hundreds of institutions around the globe, Inquiries Journal's large database of academic articles is completely free. Learn more | Blog | Submit Latest in Environmental Studies |